Research Team of Pavel Hyršl
Keywords:
innate immunity, insect immunity, entomopathogenic nematodes, eicosanoids, silkworm, Bombyx mori, wax moth, Galleria mellonella, Drosophila melanogaster, Photorhabdus bacteria, fish immunology, immunology of birds, comparative immunology
| Group leader: | assoc. prof. RNDr. Pavel Hyršl, Ph.D. |  |
| Office: | UKB – A36/123 | |
| E-mail: | hyrsl@sci.muni.cz | |
| Telephone: | 549 494 510 | |
| List of teaching | ||
| List of publications | ||
| Projects | ||
Ph.D. students:
Bachelor and Master students:
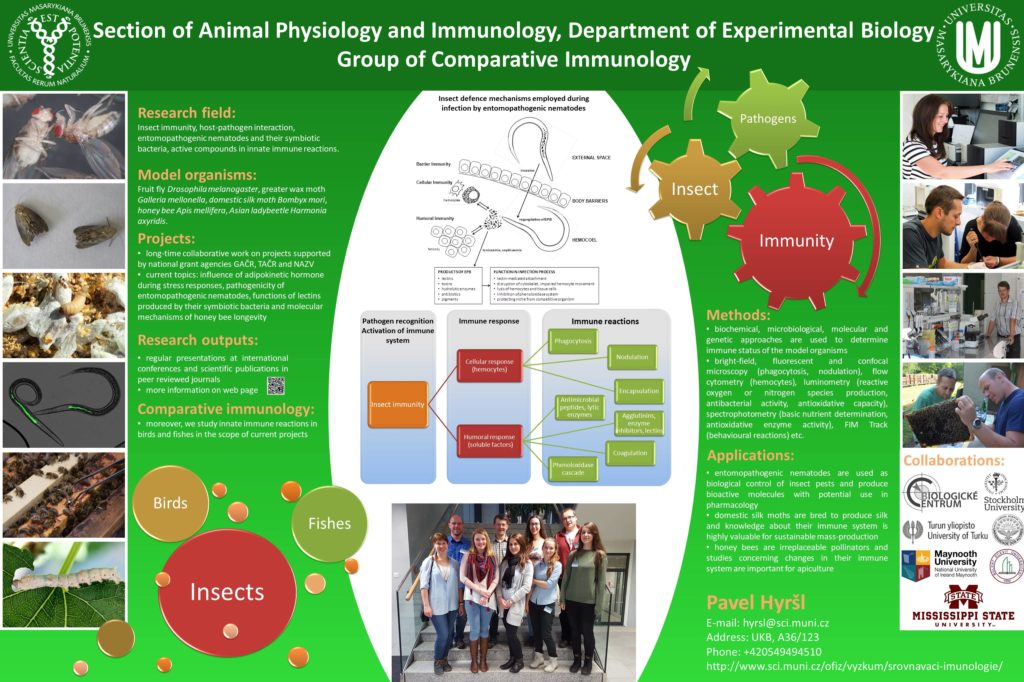
The cornerstone of defence for most of the organisms are innate immune reactions. Moreover, there is adaptive immunity in the vertebrates which is developed during life of the organism. Comparative immunology focuses on adaptations of immune system during evolution and it searches for differences or conformity in homeostatic processes and immune reactions. The innate immune reactions act as the first response to invading pathogens, it is source of antibacterial compounds, eliminate foreign particles and it is part of other immune reactions, e.g. coagulation cascade. Research conducted on insect immune system is utmost important because it can help to understand immune system of much more sophisticated vertebrates. Methods used to study innate immune reactions are similar for invertebrates same as for vertebrates; therefore, we focused on examining of immune system in insect, fish and birds (representatives of invertebrates, cold-blooded and warm-blooded organisms).
Our research is focused on insect immune system, which we chose from wide range of physiological and immune reactions. Insect immune system, which is comprised from humoral and cellular parts, is highly specialized and original way of defence against pathogens. It is different, compare to mammalian immune system; however, there are some analogous reactions. The differences between insect and mammalian immune systems are given to size of the insect, simplicity of nervous system, solid cuticle on the surface of the body and other reasons due to morphological, anatomical and physiological variances of the insect.
At first the immune responses are activated by some stressor what is followed by measurement of changes in, e.g. proteomic spectrum of haemolymph, production of free radicals, activity of antibacterial and antioxidative system etc. As the stressor can be used for example temperature variations, injury, pathogen injection, natural infection by entomopathogenic nematodes etc. Many immune responses can be influenced by exposure to hormones or chemicals like insecticides or pollutants.
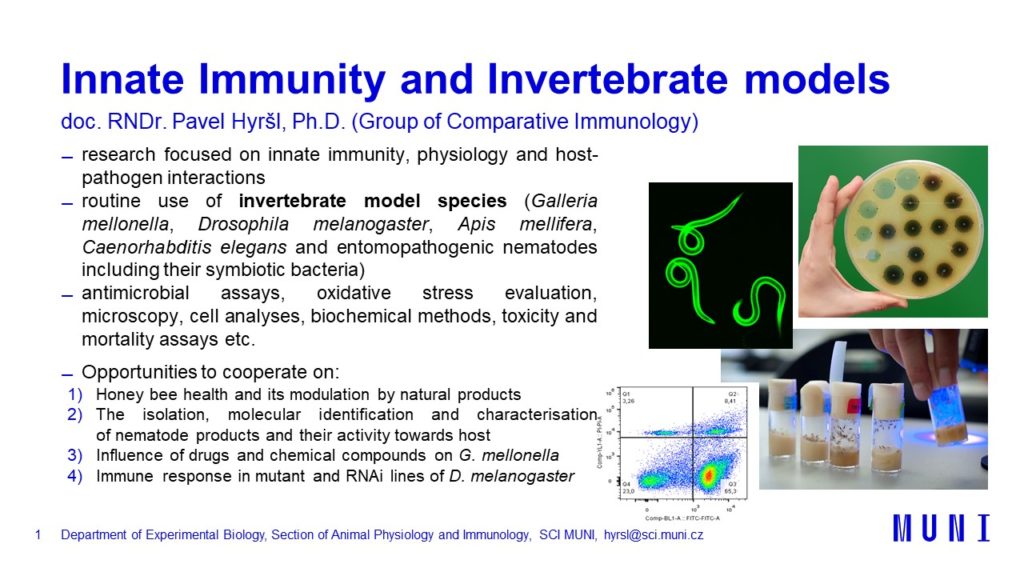
Fruit fly (Drosophila melanogaster), greater wax moth (Galleria mellonella), domestic silk moth (Bombyx mori), honey bee (Apis mellifera) or other are used as model organisms. Most of the experiments are conducted on haemolymph collected from above mentioned model organism. We employ broad spectrum of methods to determinate immune status of the organism like polyacrylamide electrophoresis, luminometric methods, biochemical kits, ELISA, PCR, confocal microscopy, flow cytometry, behavioural methods etc.
Recently, entomopathogenic nematodes have received much attention due to their possible use as biological control of insect pests. Heterorhabditis bacteriophora and Steinernema feltiae are mostly used for experiments in our group. These two species differ in pathogenicity and the hosts utilize different immune reactions in fight with the them. The experiments are mostly conducted on D. melanogaster and G. mellonella under various conditions.
Heterorhabditis bacteriophora is symbiotically associated with entomopathogenic bacteria of genus Photorhabdus forming nematobacterial complex. This bacterial genus is the only known terrestrial bacteria with natural bioluminescence activity. Luminometric detection of bioluminescence and its changes under various stressors is the basic tool to study these bacteria. The obtained knowledge can be used for example in various detections systems based on viability of bacteria.
We use natural infection of D. melanogaster larvae by entomopathogenic nematodes to study innate immune responses of the host body to bacteria released from nematodes. Various mutant or RNAi lines of D. melanogaster can be used to study which genes are involved in innate immune reactions of insects. This procedure can be employed in study of coagulation cascade, eikosanoids, antimicrobial peptides, and many other immune reactions.
Moreover, our group focus on study of honey bee longevity and differences between short-living and long-living generations in hive. Honey bee immune system, same as in other insect species, is composed from humoral and cellular part; however, honey bees possess also social immune system, which incorporate complicated behavioural patterns inside the colony leading to better efficiency of the immune responses. Measurements of physiological and immune parameter can help us to establish burden of parasites, pathogens or chemicals influencing the hive.
We cooperate with other laboratories on studies fish and bird innate immune reactions. In collaboration we measure properties of leucocytes (oxidative ignition) and antibacterial activity of plasma (complement system, concentration of lysozyme). We use several freshwater fish species (carp, trout, tench, crucian carp, roach, grayling) and birds (hens, quails, partridges, titmouse).
Current research:
- Symbiotic bacteria of entomopathogenic nematodes – genus Photorhabdus and Xenorhabdus (P. Hyršl, P Dobeš)
- Immune parameters of fresh water fish. (P. Hyršl)
- Honey bee immunity (P. Hyršl, P. Dobeš, J. Hurychová)
- The nematobacterial complex Heterorhabditis – Photorhabdus and its effect on Drosophila immunity (P. Hyršl, P. Dobeš, J. Hurychová, S. Šreibr)
- Lectins from Photorhabdus bacteria (P. Dobeš)
- Behavioural patterns of insect – FIM Track (J. Hurychová)
- Secreted/excreted products of entomopathogenic nematodes (J. Hurychová, S. Šreibr)
- Honey-bee hemocytes under stress conditions (J. Marciniak)
- Role of eicosanoids and nitric oxide in insect under influence of adipokinetic hormone (P. Dobeš)
- Interspecies variability of lady bugs (P. Dobeš)
- Effectivity of HIF-1alfa inhibitors on Ras-induced tumors in Drosophila melanogaster (M. Šindlerová)
- Bioactive molecules produced in vitro by the entomopathogenic nematode Heterorhabditis bacteriophora after contact with its insect host (A. Lázničková)
- The interaction of excreted/secreted products of entomopathogenic nematode Heterorhabditis bacteriophora with Drosophila melanogaster larvae (P. Streit)
Collaborating institutions
- Institute of Biophysics (Laboratory of Free Radical Pathophysiology) (Brno)
- Centre of Structural Biology, CEITEC (Brno)
- Institute of Vertebrate Biology, Czech Academy of Sciences (Brno)
- Institute of Botany and Zoology, Department of Parasitology (Brno)
- Veterinary Research Institute (Brno)
- Czech University of Life Sciences (Prague)
- Institute of Entomology (Laboratory of Entomopathogenic Nematodes, Laboratory of Insect Physiology) (České Budějovice)
- Bee Research Institute (Dol)
- Palacký University Olomouc (Department of Biochemistry) (Olomouc)
- Bülent Ecevit University (Department of Biology) (Zonguldak, Turkey)
- Maynooth University (Human Health institute) (Maynooth, Ireland)
- Stockholm University (Department of Molecular Biosciences, The Wenner-Gren Institute) (Stockholm, Sweden)
- University of Bari (Instituto de Entomologia Agraria) (Bari, Italy)
- University of Turku (Department of Biochemistry and Food Chemistry) (Turku, Finland)