Mutations in genes affecting primary cilia cause ciliopathies, a diverse group of disorders often affecting skeletal development. This includes Jeune syndrome or asphyxiating thoracic dystrophy (ATD), an autosomal recessive skeletal disorder. Unraveling the responsible molecular pathology helps illuminate mechanisms responsible for functional primary cilia.
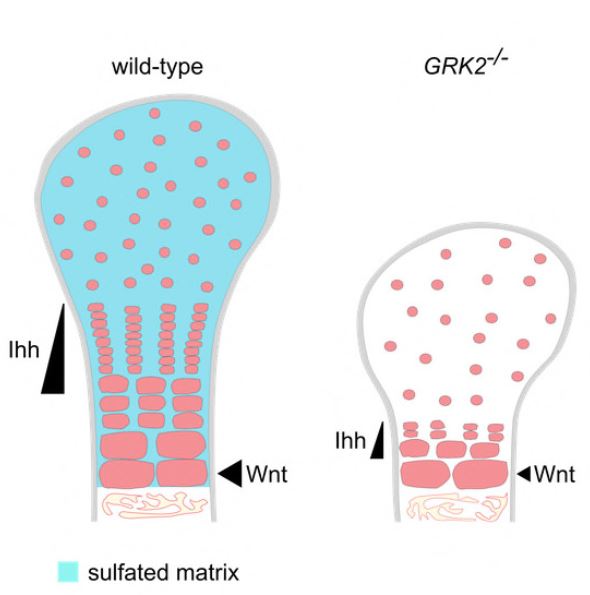
We identified two families with ATD caused by loss‐of‐function mutations in the gene encoding adrenergic receptor kinase 1 (ADRBK1 or GRK2). GRK2 cells from an affected individual homozygous for the p.R158* mutation resulted in loss of GRK2, and disrupted chondrocyte growth and differentiation in the cartilage growth plate. GRK2 null cells displayed normal cilia morphology, yet loss of GRK2 compromised cilia‐based signaling of Hedgehog (Hh) pathway. Canonical Wnt signaling was also impaired, manifested as a failure to respond to Wnt ligand due to impaired phosphorylation of the Wnt co‐receptor LRP6.
We have identified GRK2 as an essential regulator of skeletogenesis and demonstrate how both Hh and Wnt signaling mechanistically contribute to skeletal ciliopathies.
Authors:
Michaela Bosakova, Sara P. Abraham, Alexandru Nita, Eva Hruba, Marcela Buchtova, Paige Taylor, Ivan Duran, Jorge Martin, Katerina Svozilova, Tomas Barta, Miroslav Varecha, Lukas Balek, Jiri Kohoutek, Tomasz Radaszkiewicz, Ganesh V Pusapati, Vitezslav Bryja, Eric T. Rush, Isabelle Thiffault, Deborah A Nickerson, Michael J Bamshad, Rajat Rohatgi, Daniel H Cohn, Deborah Krakow & Pavel Krejci
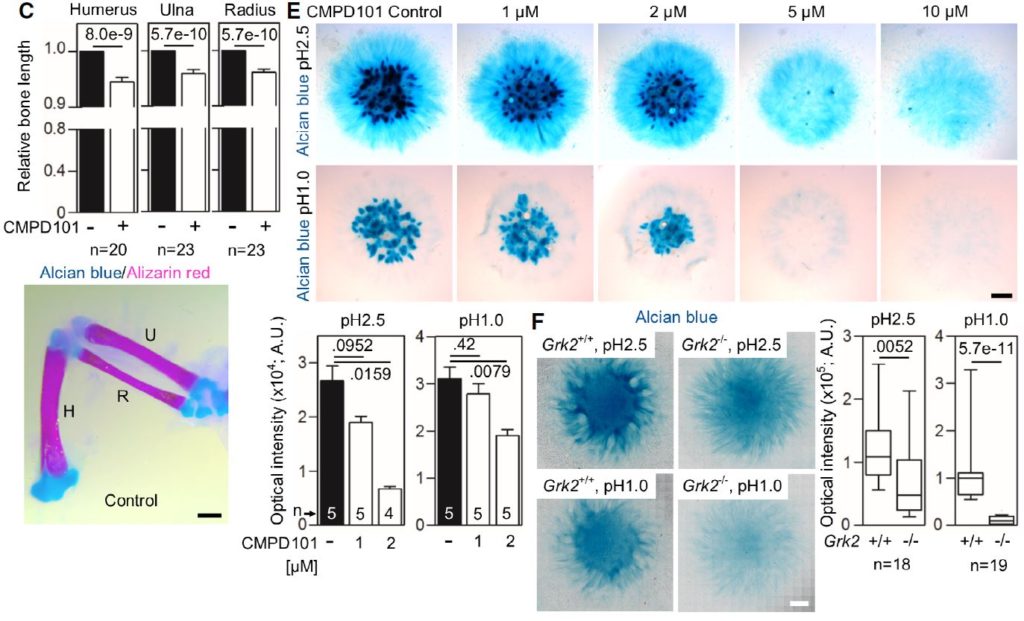
C Shortened long bones in the chicken wings injected with CMPD101. The right limb bud was injected with CMPD101, and the left one was left as a control. Note the shortened humerus (H), ulna (U), and radius (R) in the CMPD101-injected wing.
E Alcian blue staining of the chicken limb bud micromasses treated with CMPD101. Note the gradual inhibition of the cartilage nodules with increasing concentration of CMPD101, quantified below.
F Alcian blue staining of the micromasses generated from Grk2+/+ and Grk2-/- NIH3T3 cells. Note the mildly weaker staining by alcian blue pH 2.5 and the nearly absent sulfation stained by alcian blue pH 1.0 in the Grk2-/- micromasses.