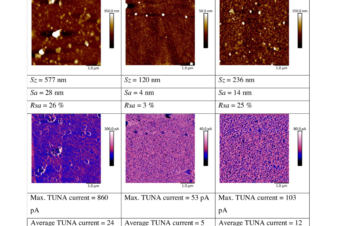
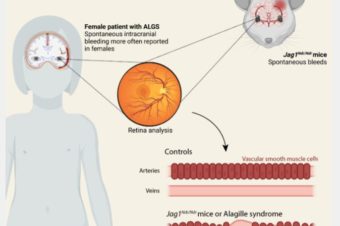
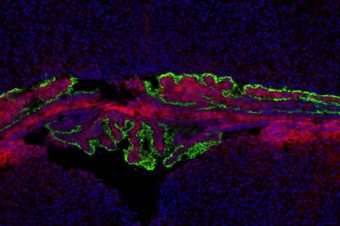
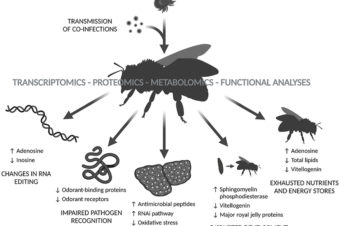
Abstract:
The PDZ domain of Dishevelled 3 protein belongs to a highly abundant protein recognition motif which typically binds short C-terminal peptides. The affinity of the PDZ towards the peptides could be fine-tuned by a variety of post-translation modifications including phosphorylation. However, how phosphorylations affect the PDZ structure and its interactions with ligands remains elusive.
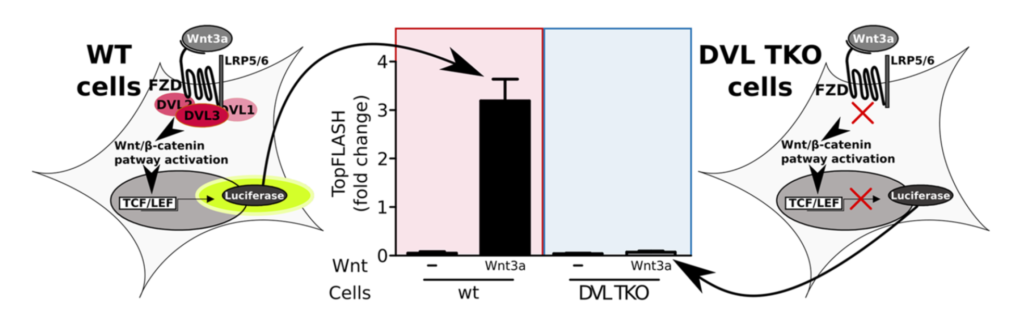
Combining molecular dynamics simulations, NMR titration, and biological experiments, we explored the role of previously reported phosphorylation sites and their mimetics in the Dishevelled PDZ domain.
Our observations suggest three major roles for phosphorylations:
(1) acting as an on/off PDZ binding switch,
(2) allosterically affecting the binding groove, and
(3) influencing the secondary binding site.
Our simulations indicated that mimetics had similar but weaker effects, and the effects of distinct sites were non-additive. This study provides insight into the Dishevelled regulation by PDZ phosphorylation. Furthermore, the observed effects could be used to elucidate the regulation mechanisms in other PDZ domains.
Sci Rep. 2021 Jan 15;11(1):1484. doi: 10.1038/s41598-020-79398-5.
Authors:
Miroslav Jurásek1, Jitender Kumar2 , Petra Paclíková3 , Alka Kumari3 , Konstantinos Tripsianes2, Vítězslav Bryja3,4, Robert Vácha5,6
1 National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Kamenice 753/5, 625 00, Brno, Czech Republic.
2 CEITEC – Central European Institute of Technology, Masaryk University, Kamenice 753/5, 625 00, Brno, Czech Republic.
3 Department of Experimental Biology, Faculty of Science, Masaryk University, Brno, 62500, Czech Republic.
4 Institute of Biophysics, Academy of Sciences of the Czech Republic, v.v.i., Brno, 612 65, Czech Republic.
5 National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Kamenice 753/5, 625 00, Brno, Czech Republic. robert.vacha@mail.muni.cz.
6 CEITEC – Central European Institute of Technology, Masaryk University, Kamenice 753/5, 625 00, Brno, Czech Republic. robert.vacha@mail.muni.cz.