Abstract:
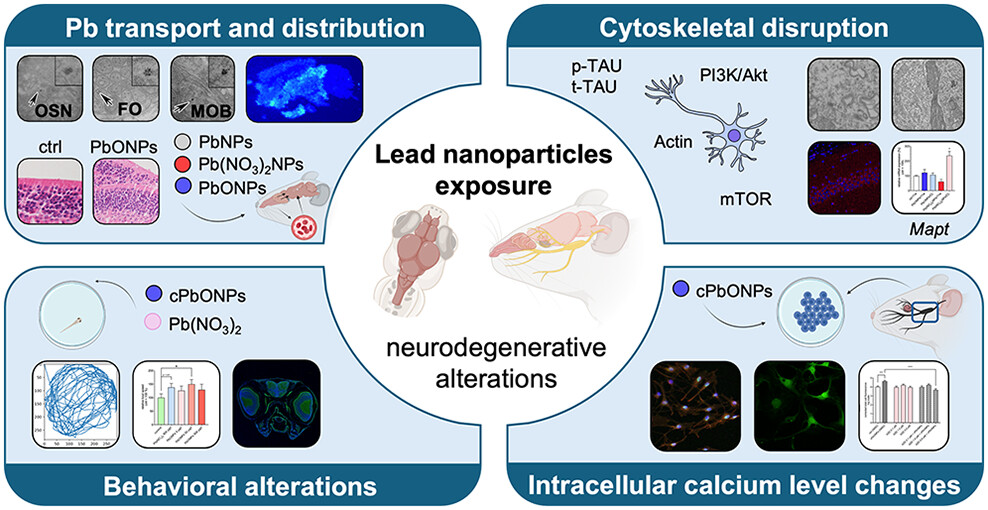
Lead nanoparticles (PbNPs) in air pollution pose a significant threat to human health, especially due to their neurotoxic effects. In this study, we exposed mice to lead(II) oxide nanoparticles (PbONPs) in inhalation chambers to mimic real-life exposure and assess their impact on the brain. PbONPs caused the formation of Hirano bodies and pathological changes related to neurodegenerative disorders through cytoskeletal disruptions without the induction of inflammation. Damage to astrocytic endfeet and capillary endothelial cells indicated a compromised blood–brain barrier (BBB), allowing PbONPs to enter the brain. Additionally, NPs were detected along the olfactory pathway, including fila olfactoria, suggesting that at least a proportion of PbNPs enter the brain directly by passing through the olfactory epithelium. PbNP inhalation severely damaged the apical parts of olfactory epithelial cells, including the loss of microtubules in their ciliary distal segments. Inhalation of PbONPs led to the rapid accumulation of lead in the brain, while more soluble lead(II) nitrate NPs did not accumulate significantly until 11 weeks of exposure. PbNPs induced disruption of the BBB at multiple levels, ranging from ultrastructural changes to functional impairments of the barrier; however, they did not induce systemic inflammation in the brain. The clearance ability of the brain to remove Pb was very low for both types of NPs, with significant pathological effects persisting even after a long clearance period. Cation-binding proteins (ZBTB20 and calbindin1) were distributed unevenly in the brain, with the strongest signal located in the hippocampus, which exhibited the greatest defects in nuclear architecture, indicating that this area is the most sensitive structure for PbNP exposure. PbNP exposure also altered the PI3K/Akt/mTOR signaling pathway, and tau phosphorylation in the hippocampus and inhibition of tau phosphorylation by GSK-3 inhibitor rescued the negative effect of PbONPs on the intracellular calcium level in trigeminal ganglion cultures. In zebrafish larvae, PbONPs affected locomotor activity and reduced calcium levels in the medium enhanced negative effect of PbONP on animal mobility, even increasing lethality. These findings suggest that cytoskeletal disruption and calcium dysregulation are key factors in PbNP-induced neurotoxicity, providing potential targets for therapeutic intervention to prevent neurodegenerative changes following PbNP exposure.
Authors:
Adriena Jedličková, Daniela Kristeková, Zuzana Husáková, Pavel Coufalík, Lucie Vrlíková, Tereza Smutná, Michaela Capandová, Lukáš Alexa, Denisa Lusková, Kamil Křůmal, Veronika Jakešová, Zbyněk Večeřa, Nikodém Zezula, Viktor Kanický, Aleš Hampl, Tomáš Vaculovič*, Pavel Mikuška*, Jana Dumková, Marcela Buchtová*