Abstract:
Mineralized tissues, such as bones or teeth, are essential structures of all vertebrates. They enable rapid movement, protection, and food processing, in addition to providing physiological functions. Although the development, regeneration, and pathogenesis of teeth and bones have been intensely studied, there is currently no tool to accurately follow the dynamics of growth and healing of these vital tissues in space and time. Here, we present the BEE-ST (Bones and tEEth Spatio-Temporal growth monitoring) approach, which allows precise quantification of development, regeneration, remodeling, and healing in any type of calcified tissue across different species. Using mouse teeth as model the turnover rate of continuously growing incisors was quantified, and role of hard/soft diet on molar root growth was shown. Furthermore, the dynamics of bones and teeth growth in lizards, frogs, birds, and zebrafish was uncovered. This approach represents an effective, highly reproducible, and versatile tool that opens up diverse possibilities in developmental biology, bone and tooth healing, tissue engineering, and disease modeling.
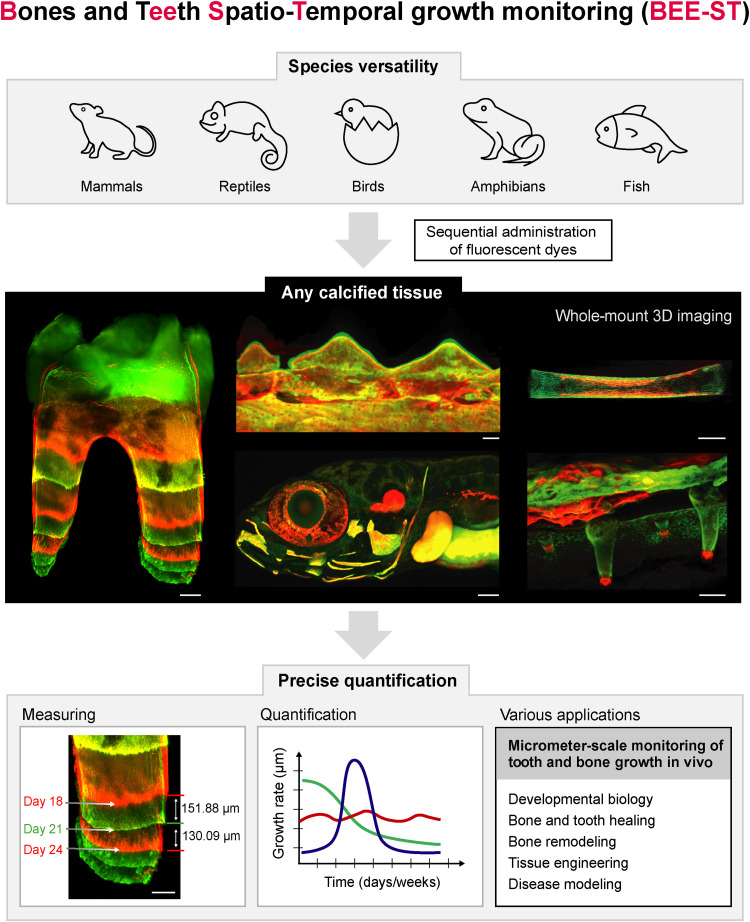
Fig. 1.. Possibilities of the BEE-ST growth monitoring technique application. We present the BEE-ST approach for tracking the dynamics of hard tissue growth in space and time. Our approach is applicable to all main groups of vertebrates represented by their model organisms: mammals (M. musculus), reptiles (C. calyptratus), birds (G. gallus), amphibians (X. laevis), and fish (D. rerio). It is based on the sequential administration of calcium-incorporating fluorescent dyes, followed by optical tissue clearing of fully calcified hard tissues, whole-mount imaging, and subsequent precise quantification of the growth in a micrometer scale. BEE-ST has many potential applications in fundamental biology, tissue engineering, or disease modeling. Scale bars, 100 μm. 3D, three-dimensional.
Authors:
Marcos Gonzalez Lopez 1 , Barbora Huteckova 2, 3 , Josef Lavicky 1 , Nikodem Zezula 2 , Vladislav Rakultsev 1 , Vendula Fridrichova 1 , Haneen Tuaima 1 , Cita Nottmeier 4 , Julian Petersen 4 , Michaela Kavkova 1, 5 , Tomas Zikmund 5 , Jozef Kaiser 5 , Rupali Lav 6 , Haza Star 6 , Vítězslav Bryja 2 , Petr Henyš 7 , Miroslav Vořechovský 8 , Abigail S Tucker 6, 9 , Jakub Harnos 2 , Marcela Buchtova 2, 3 , Jan Krivanek 1
- 1 Department of Histology and Embryology, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
- 2 Department of Experimental Biology, Faculty of Science, Masaryk University, Brno, Czech Republic.
- 3 Institute of Animal Physiology and Genetics, Czech Academy of Sciences, Brno, Czech Republic.
- 4 Department of Orthodontics, University of Leipzig Medical Center, Leipzig, Germany.
- 5 Central European Institute of Technology, Brno University of Technology, Brno, Czech Republic.
- 6 Centre for Craniofacial and Regenerative Biology, King’s College London, London, UK.
- 7 Institute of New Technologies and Applied Informatics, Faculty of Mechatronics, Informatics and Interdisciplinary Studies, Technical University of Liberec, Liberec, Czech Republic.
- 8 Institute of Structural Mechanics, Faculty of Civil Engineering, Brno University of Technology, Czech Republic.
- 9 Institute of Histology and Embryology, First Faculty of Medicine, Charles University, Prague, Czech Republic.